Understanding the Ultimate Battery Chemistry: Rechargeable Lithium/Air
A rechargeable Lithium/Air battery can potentially store ten times the energy of a Lithium/Ion battery of the same weight, making practical widespread use of fully electric cars. But realizing this enormous potential is a very challenging scientific problem. Therefore, an interdisciplinary effort from IBM Research, Vanderbilt University, Oak Ridge National Laboratory (ORNL), and Argonne National Laboratory (ANL) is focusing on this problem.
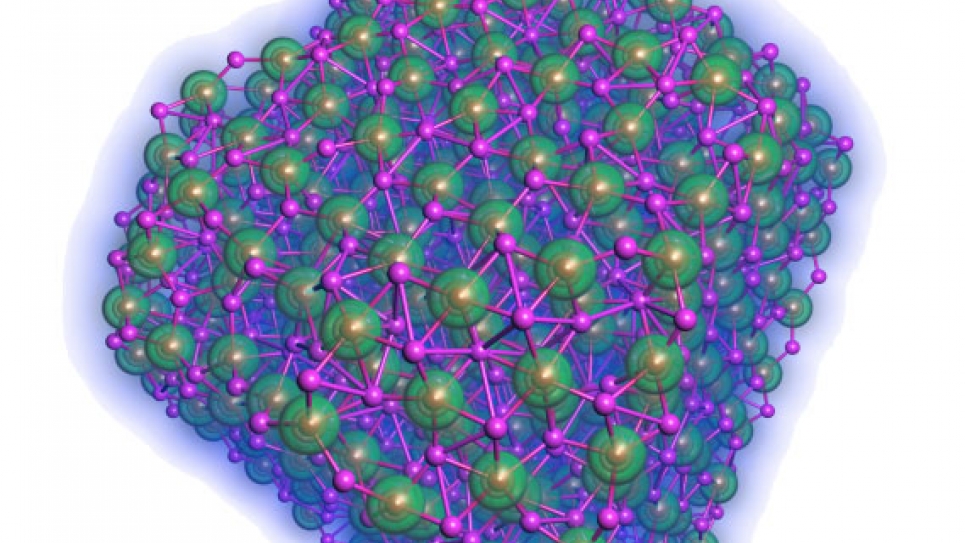
Currently, the main challenges are to realize a high percentage of the theoretical energy density, improve electrical efficiency of recharging, increase the number of times the battery can be cycled, and improve the power density. As the key reaction product in a Lithium/Air battery, various properties of Li2O2 remain elusive and needed to be explored. Thus, at Argonne, the current primary focus is on the basic system (e.g., bulk crystals, surfaces, and nanoparticles) that resides on the cathode interfaces as the Li/Air cells’ main reaction products. So far, the fundamental understanding of these systems that are governed by electronic, structural, and thermodynamic properties have been obtained by using the well-parallelized Density Functional code installed on Intrepid, the IBM Blue Gene/P system at the Argonne Leadership Computing Facility. These results will provide useful insights for the design of Li/ Air cells in solving the discharge/recharge reactions at the electrode-electrolyte interface in the future.