Vibrational spectroscopy of liquid mixtures and solid/liquid interfaces
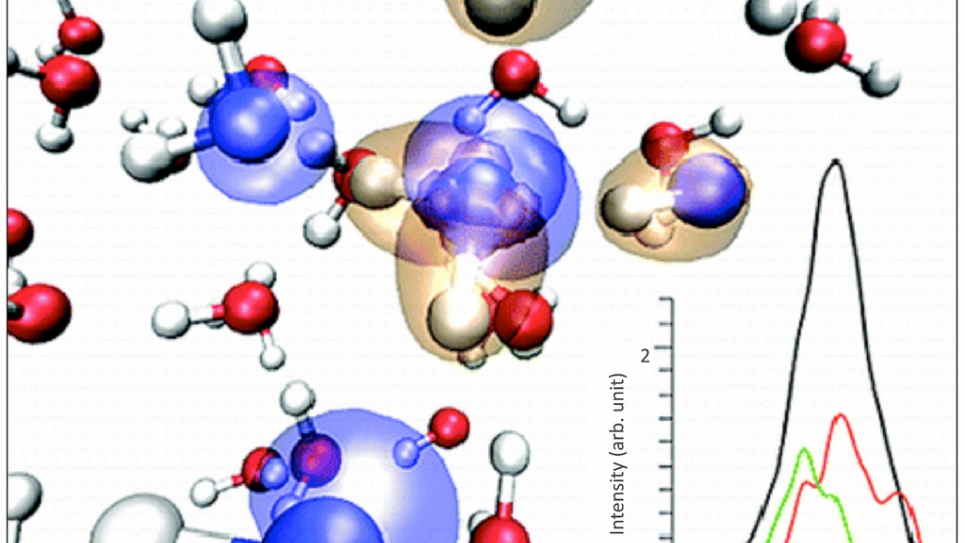
In the last several decades, vibrational spectroscopy has been widely used to probe the structure and dynamics of water, and much progress has been made in the understanding of hydrogen bonding in the liquid, based on two-dimensional (2-D) IR spectroscopy. However, despite experimental and theoretical advances (e.g., the interpretation of IR and 2-D-IR spectra provided by several simulation studies), a detailed understanding of the infrared (IR) line shapes of liquid water has not yet been achieved.
University of California—Davis researchers interpreted the complex shape of the IR stretching band of neat, heavy water, using first principles molecular dynamics and ab-initio electronic structure calculations [1]. They carried out calculations using the Qbox code on Intrepid, the IBM Blue Gene/P supercomputer at the Argonne Leadership Computing Facility. The researchers showed that intermolecular dipolar correlations play a key role in determining the shape and width of the band, and that these correlations are long-ranged, extending to the second coordination shell. Both hydrogen-bonded and non-hydrogen-bonded molecules contribute to the IR stretching band over the entire frequency range, with no distinctive peak or shoulder associated with each species. Within a molecular orbital picture, the researchers identified specific features of the band arising from correlations of electronic contributions to the IR activity. The researchers’ interpretation of the IR stretching band of water is providing a better understanding of hydrogen bonding. Additional spectroscopic investigations carried out by the UCD team, in collaboration with researchers at LLNL and MIT include the study of X-Ray absorption spectra [2].
Physics issues that remain to be explored include the effects of the presence of solvated ions on the properties of neat and confined water and the analysis of electronic spectra (e.g., x-ray absorption spectra).
[1] “First Principle Analysis of the IR Stretching Band of Liquid Water”,
C. Zhang, D. Donadio and G. Galli,
J. Phys. Chem. Lett.1, 1398 (2010).
[2] “Local Effects in the X-ray Absorption Spectrum of Salt Water”, Heather J. Kulik, Nicola Marzari, Alfredo A. Correa, David Prendergast, Eric Schwegler, and Giulia Galli, J. Phys. Chem. B 114, 9594 (2010