Getting from H2O to hydrogen fuel
Producing hydrogen, a clean-burning alternative fuel, from water and sunlight almost sounds too good to be true.
But that’s the idea behind photoelectrochemical (PEC) cells, which use solar energy to trigger a chemical reaction that splits water (H2O) into hydrogen (H2) and oxygen (O2) gases. Specialized photoelectrode materials within the PEC cells act as a catalyst to drive the energy conversion process.
Research into PEC water splitting is still in its early stages, but the promising approach could lead to technology for sustainable and clean hydrogen production. The key challenge to developing scalable, commercially viable PEC cells is identifying stable photoelectrode materials that efficiently absorb solar energy and catalyze the water splitting process. Making the task even more difficult, the materials must also be cost-effective, earth-abundant, and non-toxic.
To help accelerate research and development efforts, Giulia Galli, a professor at the University of Chicago’s Institute for Molecular Engineering, is leading a project at the Argonne Leadership Computing Facility (ALCF), a DOE Office of Science user facility, to advance the understanding of PEC water splitting.
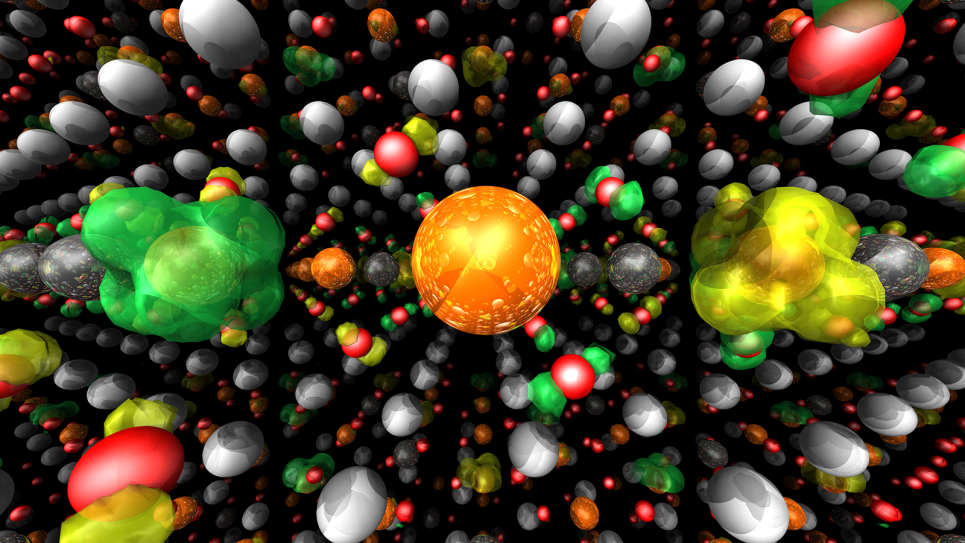
Galli is carrying out large-scale simulations on Mira, the ALCF’s 10-petaflops IBM Blue Gene/Q supercomputer, to model the physical and chemical processes occurring at the interface between solid photoelectrodes and electrolytes (water with dissolved salts, acids, and bases) at the microscopic scale. Her research team, in collaboration with Professor Francois Gygi at the University of California, Davis, is using a set of simulation programs as a computational spectroscopy tool to probe and predict vibrational and electronic properties at the solid-liquid interface.
“To understand how PEC cells work, you need to understand what’s happening at the interfaces between solutions and photoelectrodes, how light is absorbed, and how the system reacts after it is absorbed,” Galli said. “This knowledge can be used to come up with design rules that help predict the best photoelectrode materials to use for PEC cells.”
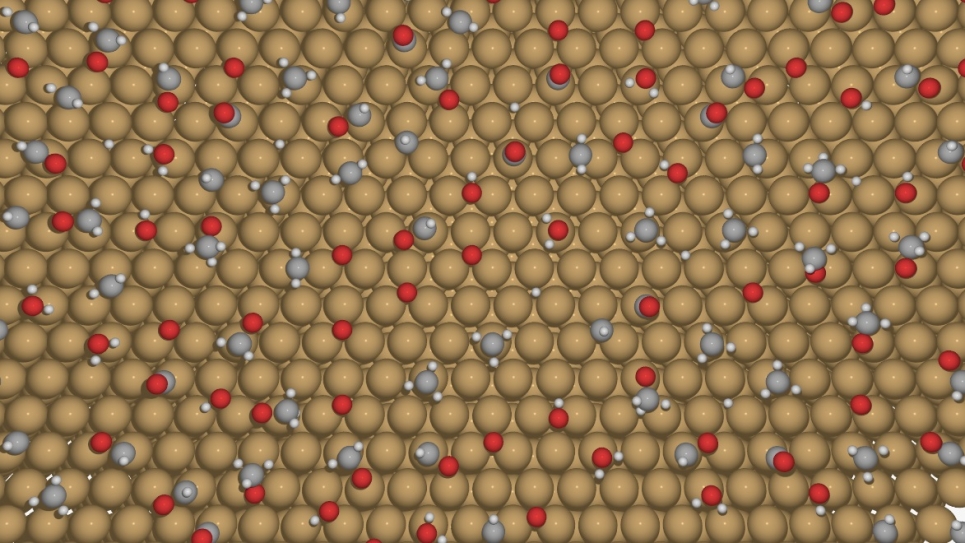
Galli’s team has recently published several papers on their studies of photoelectrodes and the electronic properties of water, which are critical to understanding interfacial properties. In their latest publication, a cover article in Chemical Physics Letters, Galli and her colleagues focused on studying salt (NaCl) solutions using ab initio molecular dynamics simulations.
The researchers implemented hybrid functionals into the Qbox code (the program written by Gygi and used for the team’s first principles simulations) to study the solution’s electronic properties and accurately account for the properties of anions. This approach, which was validated through comparisons with experimental results, allowed them to carry out simulations with the functionals with controllable accuracy.
Performing large-scale simulations with hybrid functionals is a computationally expensive task that could only be accomplished on a massively parallel supercomputer like Mira. Galli's team used as many as 16 of Mira's 48 racks, or more than 260,000 of Mira's 786,432 cores, as part of this effort. To put this into perspective, the average simulation at the ALCF runs on 8 racks, or about 131,000 cores, of Mira.
The simulations produced improved properties of solutions with respect to generalized-gradient approximations and predicted qualitatively correct positions of the energy levels of the ions with respect to the valence band of water. Among the team’s key findings was showing that the electronic properties of the chloride anion in the presence of sodium counterions (1 M concentration) are the same as those obtained at infinite dilution.
“Despite salt in water being such a common system, the properties of its electronic state have remained a mystery for a long time,” Galli said. “We need to know these electronic properties because the interfaces in PEC cells interact with solutions, not pure water.”
Galli’s work at the ALCF dates back to 2006 when she began using the facility’s supercomputers to study the properties of water and aqueous solutions with computing time awarded through DOE’s Innovative and Novel Computational Impact on Theory and Experiment (INCITE) program.
While her current INCITE award of 70 million core-hours is focused on PEC energy conversion, the methodologies and processes resulting from this work are also relevant to other research efforts involving solid-liquid interfaces, such as batteries.
In addition, results from Galli’s studies at the ALCF are helping to advance the fundamental understanding of hydrogen-bonded systems and related experiments ongoing at DOE X-ray light sources such as Argonne’s Advanced Photon Source and the SLAC National Accelerator Laboratory.
“The experiments conducted at light sources are very complicated and can be difficult to interpret without collaborations with theoretical and computational efforts,” Galli said. “We work closely with experimentalists to provide tools to help them interpret their complex measurements and possibly plan and devise new experiments.”
Argonne National Laboratory seeks solutions to pressing national problems in science and technology. The nation's first national laboratory, Argonne conducts leading-edge basic and applied scientific research in virtually every scientific discipline. Argonne researchers work closely with researchers from hundreds of companies, universities, and federal, state and municipal agencies to help them solve their specific problems, advance America's scientific leadership and prepare the nation for a better future. With employees from more than 60 nations, Argonne is managed by UChicago Argonne, LLC for the U.S. Department of Energy's Office of Science.