Lithium-Air: the "Mount Everest" of Batteries for Electric Vehicles
Is it possible to produce a novel lithium-air battery that could drive a car 500 miles per charge? Researchers from Argonne and Oak Ridge leadership computing facilities and IBM Research think so.
Weight-for-weight, a rechargeable lithium-air battery can store five to ten times the energy of a conventional lithium-ion battery. It has the highest energy density of any battery yet devised. Currently, the batteries in electric cars can’t compare with gasoline in the amount of energy derived from a given weight of fuel. Cars with lighter, more powerful lithium-air batteries could overcome this challenge. The battery’s enhanced energy storage capability would enable the widespread use of electric vehicles.
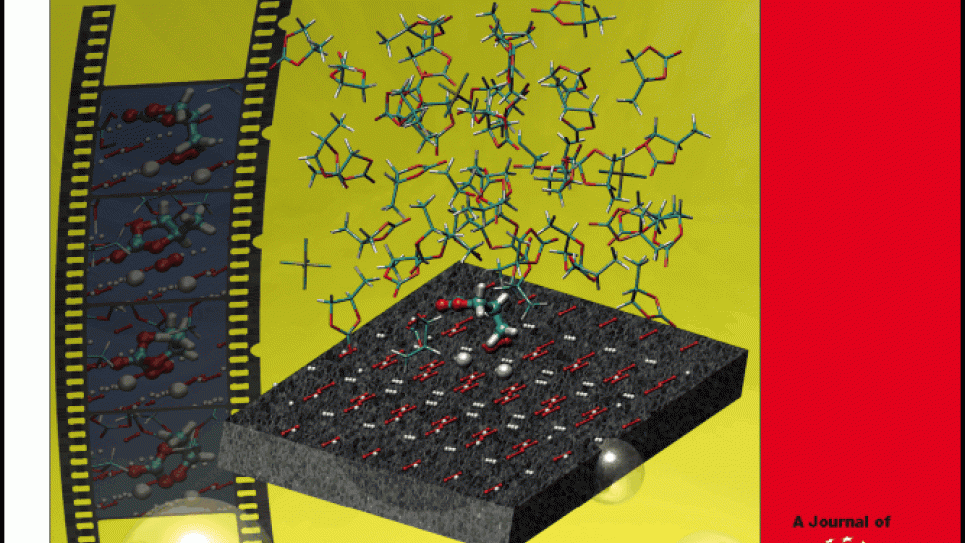
Realistic computational chemistry models are of crucial importance to developing a viable lithium-air battery. Led by Jack Wells, director of science at the Oak Ridge Leadership Computing Facility, a research team modeled how the lithium-air cell would work. Using supercomputing resources allocated by a Department of Energy INCITE award, the researchers studied the cell’s basic chemistry, how catalysts affect its performance, and how to make the device more stable. They investigated the use of propylene carbonate (PC) as a potential electrolyte for a rechargeable lithium-air battery to establish the practicality of PC. By employing sophisticated models for both the surface and the electrolyte, they achieved sufficient detail to study the full complexity of propylene carbonate degradation by lithium-air discharge products. The team evaluated this problem using three application codes -- CP2K, CPMD, and VASP -- on Intrepid, the IBM Blue Gene/P system, at the Argonne Leadership Computing Facility (ALCF).
For the first time, the scientists demonstrated that propylene-carbonate is not a suitable electrolyte for lithium-air-based batteries. Formerly, PC was believed to be stable, based on the behavior of similar systems. However, the simulations in this project revealed that during discharge, oxygen reduces to peroxide, forming layers of Li2O2 that immediately degrade PC. Moreover, special properties of the surface of Li2O2 act to enhance its reactivity. The findings also explained the recent failure to identify Li2O2 in the discharge products on the cathode surface during experimental research into this technology and determined the major chemical mechanisms behind the charge/discharge processes. The calculations that resulted in these insights used 26.5 million core-hours on Intrepid.