Studies of Large Conformational Changes in Biomolecular Machines
In biology, proteins, nucleic acids, and carbohydrates can be considered complex “molecular machines” that consume energy in order to perform specific biological functions. Their concerted actions trigger many of the critical activities that occur in living cells. In particular, membrane-associated proteins play essential roles in controlling the bidirectional flow of material and information. These vital proteins change shape and go through many conformational states to perform their functions. However, malfunctioning proteins can result in various diseases such as cancer, epilepsy, or cardiac arrhythmia.
To understand how membrane proteins operate and how they are affected by disease, researchers need detailed knowledge about all of the relevant conformational states, as well as the free energy changes that connect them. This INCITE project aims to gain a deep mechanistic perspective on membrane protein function, linking structure to dynamics by characterizing the free energy landscape that governs functional motions.
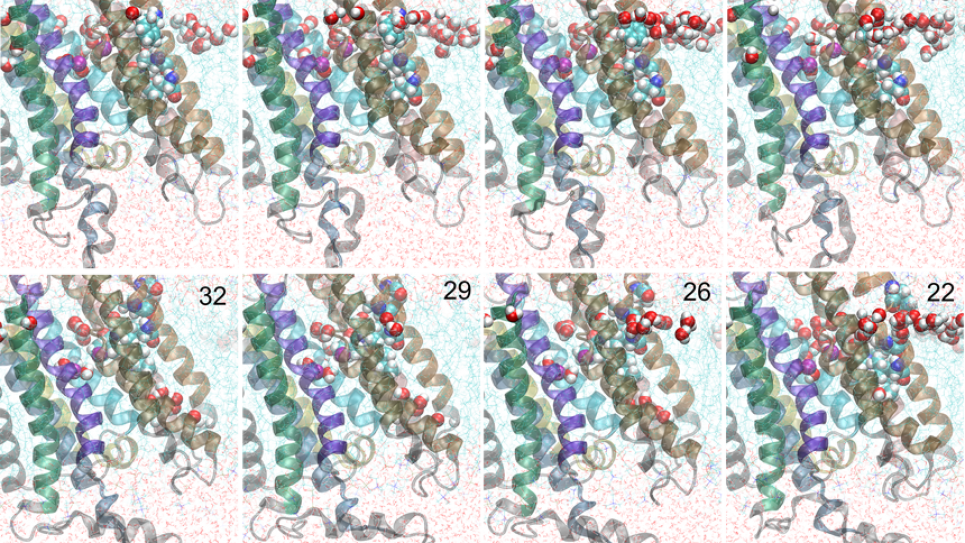
Within the unified computational perspective provided by free energy landscapes, two membrane proteins of increasing complexity and size were considered. In its third year, the project is focusing on the four remaining critical conformational transition pathways needed to describe the transport cycle of a P-type calcium pump called SERCA (pathways for two transitions have been described in the prior two years). To determine these conformational transition pathways, researchers leverage the NAMD/Charm++ with MPI-level, multiple-copy algorithms with the String Method — a computational methodology that achieves extreme scalability on leadership-class supercomputers and is at the forefront in the field of biomolecular simulations. The researchers, by studying experimentally well-characterized systems of increasing size and complexity within a unified theoretical framework based on free energy landscapes, are advancing theory-modeling-simulation (TMS) technology, which offers a virtual route for addressing fundamental biological questions such as rational protein design. Computations performed for this study are serving as a roadmap for simulating, visualizing, and elucidating how biomolecular nano-machines work.