Coding the cracks
Researchers use Mira to study stress-corrosion cracking in silicates
Encased in the rocks that comprise much of Earth’s crust and mantle are the metals and minerals we rely on to sustain modern life and technology. Silicates, like quartz, are the most abundant of these materials.
According to the World Business Council for Sustainable Development, some five percent of total human energy consumption is currently spent breaking quartz and other silicates to retrieve much-needed metal ores. Silicates also are used widely in the manufacture of products as diverse as biomedical implants and semiconductors. But because these are brittle materials, they are prone to stress-corrosion cracking which can lead to costly damage and, eventually, product failure.
At the Department of Energy’s Argonne National Laboratory, a research team from King’s College London, led by British physicist James Kermode, has been working with the Argonne Leadership Computing Facility (ALCF) on a hybrid multiscale simulation program to virtually get inside this fracturing process.
Determining how the cracks are caused will help researchers and engineers put the process to good use or stop it altogether.
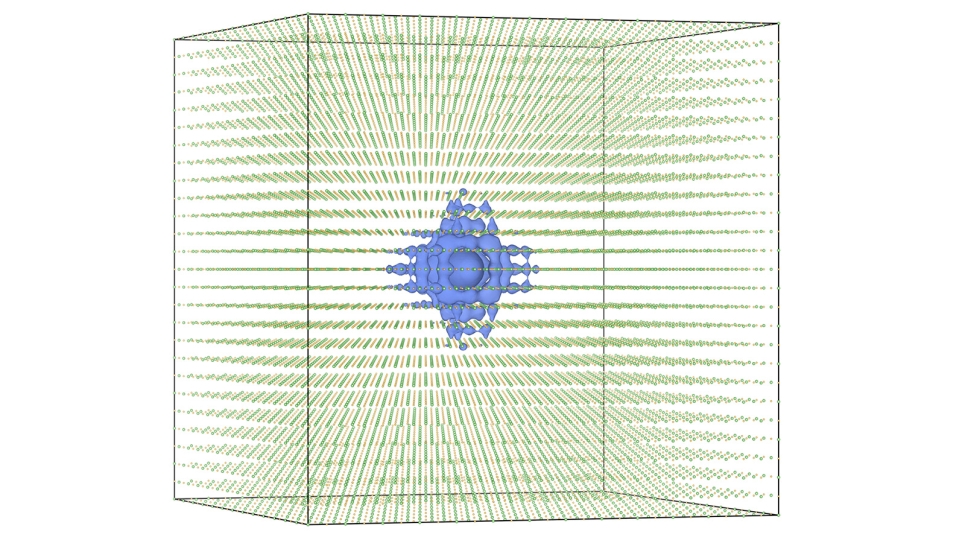
Until recently, attempting such a study had been difficult at best, both for its prohibitive costs and because of the intimate relationship between the chemical and mechanical processes, or chemo-mechanical coupling, required for the cracking to occur. Utilizing a state-of-the-art quantum mechanical/molecular mechanical (QM/MM) simulation approach, researchers have now begun to disentangle those chemo-mechanical reactions to get a closer look at the silica cracking process.
The effectiveness of this program is greatly enhanced when combined with ALCF-supported tools, such as the supercomputer Mira, an IBM Blue Gene/Q, and a machine learning method called Learn on the Fly (LOTF). Not a seat-of-the-pants proposition as the name might imply, LOTF stores data that has already been observed and uses it later to infer the solution to similar or recurring events, which dramatically speeds up a simulation.
“Through these combined methods, we envision that this project will deliver the first series of efficient QM-accurate 3D simulations of catastrophic and stress-corrosion-induced fracture in crystalline and amorphous silica,” says Kermode.
The cracking of a material is a macroscopic phenomenon, something you can see with your eyes, and these types of cracks tend to propagate very slowly. The speed of the crack is determined by the breaking of chemical bonds at the atomic level, where the atoms move much faster than any macroscopic behavior. In fact, it happens on a level nine orders of magnitude smaller than phenomena observable by the naked eye.
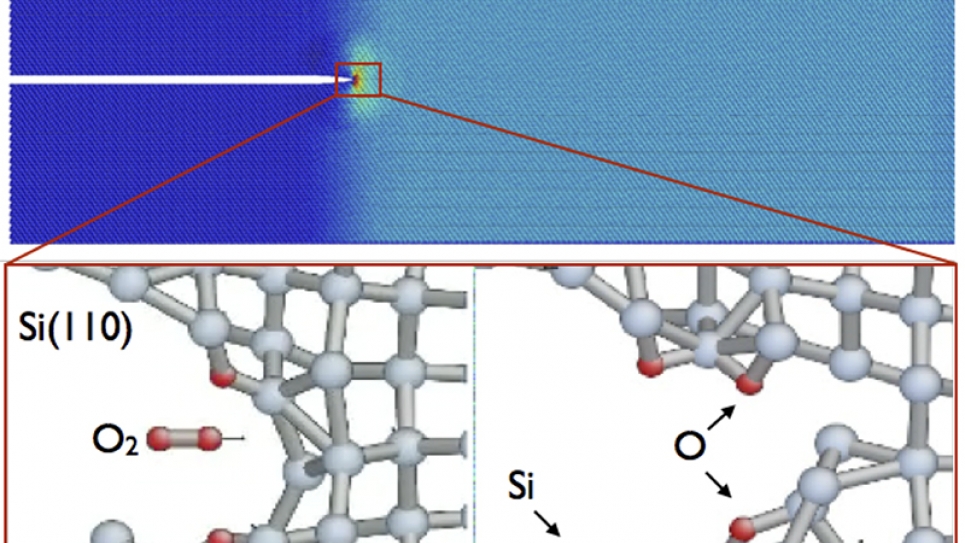
”The QM/MM program couples electronic and mechanical events on a nanoscopic scale and explains them through atomistic simulations,” Kermode explains.
To study the interaction between oxygen atoms and a silicon surface requires enormous computing power. The largest calculations could involve calculating quantum mechanical forces on over 700 atoms for millions of time steps. But where Mira is able to calculate and solve these processes in a single day, standard high-performance computing clusters typically available to university researchers could take six months of continuous calculating.
In a paper recently published in the journal Physical Review Letters, the results of the team’s simulations showed that the presence of oxygen induces a corrosive environment that makes it easier for the crack to grow. These results were then confirmed by experiments that showed no evidence of cracking in oxygen-free conditions.
The stress-corrosion process involves oxidation reactions in various types of matter, including silica materials. “It’s easier to understand if you imagine that the oxygen provides extra energy which ’burns’ parts of the material near the crack tip, breaking chemical bonds and causing the crack to move forward,” says ALCF’s Anatole von Lilienfeld, a computational scientist working on the project.
Understanding this phenomenon presents something of a two-fold result. Learning how to control the mechanisms that cause cracks could lead to increased resistance in and, thus, reduced fracturing in fragile biomaterials, like dental implants. On the other hand, enabling the process would have its own advantages.
“There are different materials to which you could apply this methodology,” notes Lilienfeld. “In this study, they looked at the effects of oxygen, but you could study the effect of carbon dioxide or other reactive small molecules. If you wanted to crush rocks more effectively, you could introduce a gas stream of more reactive molecules over the rocks before you started crushing them. This kind of technology could reduce energy expenditure dramatically.”
This work was supported by the Rio Tinto Centre for Advanced Mineral Recovery based at Imperial College, London and from the EPSRC HEmS Grant No. EP/L014742/1.
The project received computing time at the ALCF through DOE’s Innovative and Novel Computational Impact on Theory and Experiment (INCITE) program. The ALCF is a DOE Office of Science User Facility.
The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov